Introduction
The appearance of an unexpected, unexplained peak in a chromatogram during product testing strikes dread in analytical labs across the pharma and medical device industries. While the emergence of a persistent impurity peak is worrying, those that appear intermittently in some samples but not others can be even more baffling and difficult to explain. The PMBRC at SETU Waterford has worked on many impurity identification projects over the years with a good deal of success, although we have been left stumped on one or two occasions. The following blog describes how we go about tackling impurity identification projects for our industry partners.
Impurities in HPLC Chromatograms
An impurity often shows up as an unknown peak in a HPLC chromatogram. A simple way to check if it is an API degradation product is to check the PDA spectrum of the impurity and compare it to the API. Beware however: dissimilar spectra do not necessarily mean they are not related. Figure 1 compares an API spectrum with that of a drug product impurity. The impurity is a degradation product and is structurally quite similar to the API, but clearly has a very different UV spectrum.

Figure 1 PDA UV spectrum of the API (left) and API degradation product (right). Although structurally quite similar, they have very different spectra.
If API forced degradation and excipient compatibility studies have already been performed, then it may be possible to determine if this is a drug degradation product based on the relative retention time (RRT) of the impurity. An inspection of the API chromatograms following exposure to extreme temperature, light, pH and oxidation conditions may show an impurity at a similar RRT and indicate the source of the impurity. Again, a simple comparison of PDA spectra (if available) will help confirm this. However, forced degradation studies often yield messy chromatograms with many coeluting peaks and similar spectra. Often mass spectrometry will be required to definitively identify the unknown.
Liquid Chromatography Mass Spectrometry (LC/MS) analysis for unknown identification
MS is a powerful technique for determining the molecular weight of compounds eluting from a HPLC column. However, it can sometimes be hard to directly compare HPLC-UV and LC/MS chromatograms. Many industry HPLC techniques use non-volatile buffers in the mobile phase. Phosphate buffer for example has multiple pH buffering ranges and works particularly well in mobile phases, yielding good peak shape and separation resolution. Phosphates are totally incompatible with MS however and the first step in most MS projects involves the replacement of phosphate with a volatile buffer alternative such as ammonium formate. This inevitably results in a shift in retention time (and relative retention time) of the peak of interest. Finding the impurity peak in the new chromatogram can sometimes be challenging, but again, a peak area and PDA spectral comparison can help.
Assuming we can target the peak of interest, the next step is to obtain its mass spectrum if possible. Our new Q-TOF LC/MS, which was funded by an Enterprise Ireland infrastructure grant, allows high resolution MS analysis which can yield the empirical formula of the unknown compound. If the unknown is related to the API or is a known impurity/degradation compound, this can sometimes be enough to tentatively identify the structure pending confirmation via a retention time match with a standard (if available). However, if it is an exogenous impurity an empirical formula will often not be enough to identify the compound. As molecular weight increases, the number of potential structural isomers with a given empirical formula grows enormously. At this point, some structural information can be gleaned from LC/MS-MS techniques.
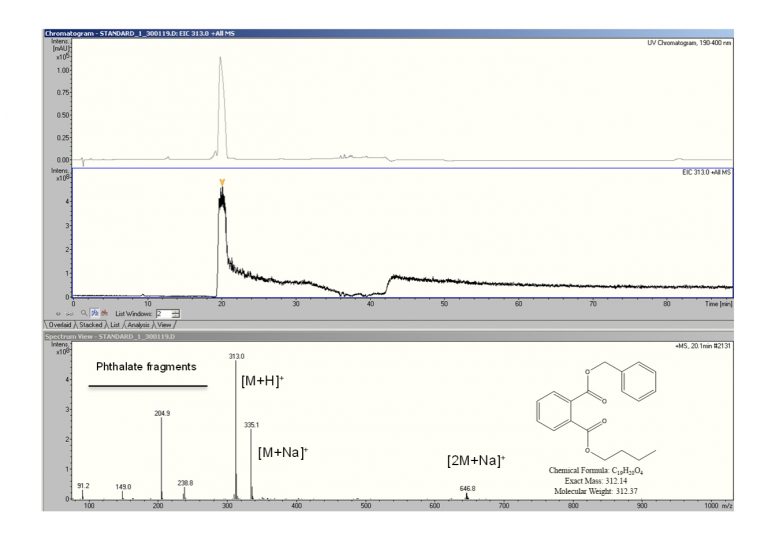
Figure 2 LC/MS analysis of a phthalate impurity. Top: UV chromatogram of the impurity; Middle: extracted ion chromatogram for the phthalate molecular ion; Bottom: mass spectrum of the impurity.
LC/MS-MS
Instead of basing the identity of an unknown compound only on its molecular weight, we can get additional information on the molecular structure using a process known as MS-MS. Basically this involves detecting the molecular mass of the compound and setting the instrument to apply a collision energy to break up the molecule into smaller fragments. These fragments are then detected again by the MS instrument. The way a molecule fragments can be used to help piece the structure of the compound together. The way a molecule fragments, the mass of the fragment ions, the relative abundances of the fragment ions and the exact molecular mass of the complete structure can be used to support the identification of an unknown compound.
Gas Chromatography Mass Spectrometry (GC/MS) Techniques
GC/MS can be a great way of identifying impurities if (and it’s a big if) they are volatile and thermally stable. Peaks in GC tend to be very narrow and well resolved from other components. Electron impact (EI) ionisation causes fragmentation of the unknown molecule and the resulting fragmentation pattern can be matched against a database to help identify the compound. Structurally similar compounds or those in the same homologous series will have similar fragmentation patterns which can be hard to distinguish from each other. However, it can be useful to determine the general class of compound (e.g. phthalates) while chemical ionisation (CI), which causes less fragmentation, can be used to identify the molecular ion.
Summary
LC/MS and GC/MS, aided by knowledge of degradation pathways and potential contamination sources, can be very powerful tools in the identification of unknown impurities which show up during analytical testing. These projects are rarely straightforward and success is not guaranteed. However, with a bit patience these unknowns and their source can often be identified.
Authors:
Niall O’Reilly, Ph.D., PMBRC Technology Gateway Manager
Mike Kinsella, Ph.D., Lecturer in Pharmaceutical Science, SETU
