Development of an electrical drug delivery patch
Product development
Design+ engaged with Magnetar Medical Devices to further design and develop their product. The electrical drug delivery patch (patent pending) has a niche application as a medical product, replacing traditional hospital IV infusion equipment used to deliver morphine to patients. The product is designed to be initially applied by a medical practitioner onto the patient for the first delivery of the drug and then used by the patient thereafter.
Safe application
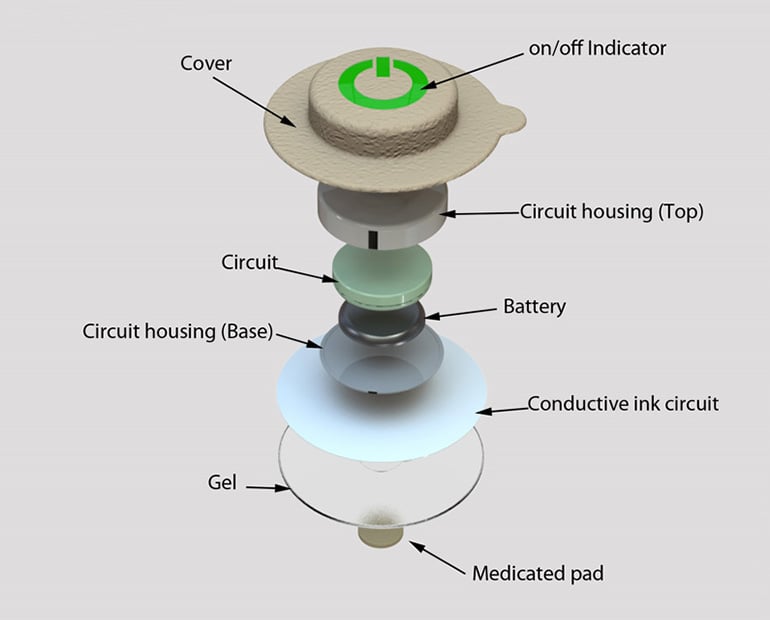
The delivered design of the product has many key features that allow the product to be used successfully and safely by the patient and medical practitioner. Some of these features include a boost button, LED interaction points and the ability to refill the product. The patch was visually designed by Design+ to conceal its presence by making the material the same colour as skin tone; over-the-counter plasters use this technique to great effect.

72
hours of IV morphine infusion
$6
billion mature market
1
Innovation Voucher
“We have found the Design+ Technology Gateway input to be inspirational and efficiently executed. Incorporating their know-how at an early stage has expedited the process of fully realising our concepts. We are extremely satisfied with the work produced and found Design+ to be responsive, dynamic and creative.”
Ger Fitzgibbon
Magnetar Medical Devices
The Magnetar Medical Devices & Design+ partnership
Once used, the patch can be easily removed by pulling a tab, and then refilled. The whole system and product was designed to be simple, manufactured efficiently and use as little material as possible while still being easily modified if required. The project combined design thinking with electronic engineering.
On conclusion of the project, the client was provided with a set of technical drawings of the product components and assemblies along with corresponding 3D computer generated models. They were also supplied with materials and manufacturing specifications. Since their engagement with Design+ the client has subsequently engaged with potential investors.
Magnetar Medical Devices are currently operating from the Institute’s Enterprise and Research Incubation Campus in Carlow. They were selected as one of the best technologies in the EU at the Medtec 2016 Europe exhibition. They have also successfully secured Competitive Start Funding from Enterprise Ireland.