What is Raman Spectroscopy
One of the primary technical areas of expertise at CAPPA is spectroscopy. Spectroscopy, in a broad sense, is the study of the interaction between radiation and matter as a function of wavelength. CAPPA’s spectroscopic platform is primarily based on Fourier Transform Infrared (FTIR) and Raman spectroscopy techniques and its capabilities over the electromagnetic spectrum range from the ultraviolet, through the visible, near IR and mid IR and beyond to the Terahertz region. Raman spectroscopy is a widely used technique applicable in the study of Carbons, Nanomaterials, Forensics, Life Sciences, Minerals, Geology and Gems, Pharmaceuticals and Polymers. Raman scattering is based upon the interaction of light with the chemical bonds within the sample. Raman microscopes utilise the combination of imaging and spectroscopy to produce three – dimensional data cubes, that transforms a standard microscopic image into a chemical image. CAPPA has two Raman Confocal Imaging Spectrometers with 532 nm, 633 nm, 785 nm and 830 nm laser lines. Raman spectroscopy is a non-contact, non-destrictive, method of elucidating the composition and structure of unknown materials without sampling handling, processing, or use of reagents which makes it ideal for use in the pharmaceutical industry.
Outcomes
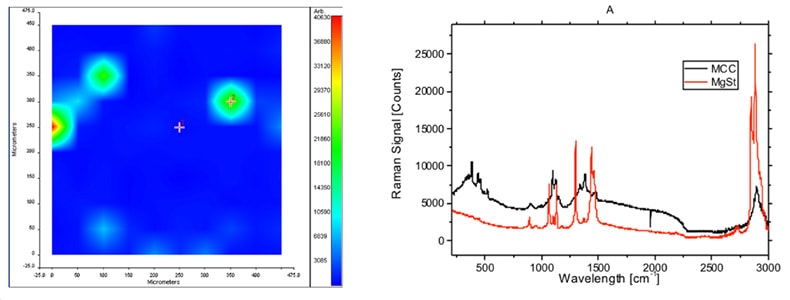
Raman spectroscopy in the pharmaceutical industry can be used for a variety of testing, including verification of raw materials, monitoring of counterfeit drugs, product shelf life, process monitoring of drug production and monitoring of quality control of products as well as providing further detail into a variety of issues. One application of Raman spectroscopy for the pharmaceutical industry is in mapping to measure a pharmaceutical blend. Such a measurement can be used for quality control and also for counterfeit detection. The below image shows a map showing blending of a compound on the left and on the right a reference spectra of the blend.
In another such example, Raman spectroscopy was employed to investigate the interaction between two variables within a medical device used for delivering medicine into the lungs. Raman spectroscopy was used to determine why there was an initial decrease in drug load for the initial uses of a medical device. It was found there were deposits of lubricant inside the pharmaceutical product that trapped the product administered by the device. Raman spectroscopy was also able to determine that there was no change in the drug form and that the lubricant was simply encapsulating the powder and trapping it.
Expertise
In this example, CAPPA used their expertise in Raman spectroscopy combined with their vast experience in the pharmaceutical industry to deliver the results and potential solutions for the client. CAPPA’s spectroscopy capabilities can be used in sectors such as pharmaceutical, medical, photonics, food, and beverage. CAPPA staff have over 10 years of experience in Raman spectroscopy across a variety of industries within the pharmaceuticals industry. CAPPA has used Raman spectroscopy in the pharmaceuticals sector to conduct product analysis such as drug identification, raw material classification and blend uniformity, to monitor processes such as phase changes and process analytical technologies and to monitor quality control including cleaning verification and particle size analysis of powders.
You can learn more about the spectroscopy capabilities at CAPPA here and CAPPA’s work in the pharmaceuticals industry here.
