What is Spectroscopy?
Spectroscopy, in a broad sense, is the study of the interaction between radiation and matter as a function of wavelength. CAPPA’s spectroscopic platform is primarily based on UV absorption, fluorescence, Raman and Infra-Red spectroscopic techniques and its capabilities over the electromagnetic spectrum range: from UV through to visible, near-IR and mid-IR and beyond to the Terahertz region.
What is DUV Raman and Fluorescence Spectroscopy?
Raman spectroscopy is a non-contact non-destructive, method of elucidating the composition and structure of unknown materials without sample handling, processing or use of reagents. For molecules having its maximum absorption in the excitation wavelength, the intensity of Raman scattering can be enhanced by resonance. For most complex molecules like Active Pharmaceutical Ingredients (API) and excipients, the maximum absorption is in the deep UV, and hence there is always a chance for observing intense Raman scattering when a deep UV source is used, known as Resonance Raman scattering. Fluorescence spectroscopy uses light to excite the electrons in molecules of certain compounds, and as a result, the molecule emits light. Deep UV excitation invokes fluorescence from a variety of samples (Figure 1). The excitation using deep UV is highly sensitive since most of the pharmaceutical products have their absorption in deep UV.
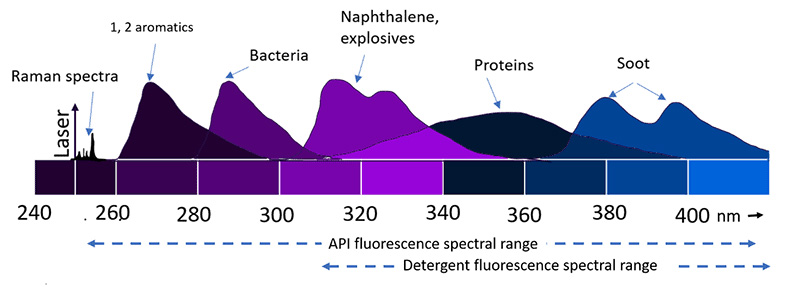
Figure 1: Representation of fluorescence and Raman from different organic compounds upon using deep UV excitation
Cleaning Validation
Cleaning validation is a required activity within the pharmaceutical and biological industries. It has a massive impact on plant efficiency, utilities and resources. In a few instances, about 50% of the time is spent engaged in cleaning, resulting in a massive impact on downtimes, costs and changeovers. This loss in time has become more problematic in recent years with an increasing focus on smaller production volumes resulting in increased numbers of changeovers and more time spent devoted to cleaning. Cleaning validation is an important activity to eliminate product cross-contamination to ensure patient safety and product quality and forms an essential component of Process Analytical Technology (PAT). Cleaning validation is an on-going activity within cGMP environments, which necessitates the investment of significant resources and time. Current analytical tests for validation such as High-Performance Liquid Chromatography (HPLC) or Ultra High-Performance Liquid
Chromatography (UPLC), Total Organic Carbon (TOC) and conductivity, requires significant resources and are time – consuming, thereby leading to an unwanted increase in production downtime. Hence, direct onsite surface measurement is highly desirable. A solution is the use of Deep Ultra Violet (DUV) excited Raman and fluorescence spectroscopy.
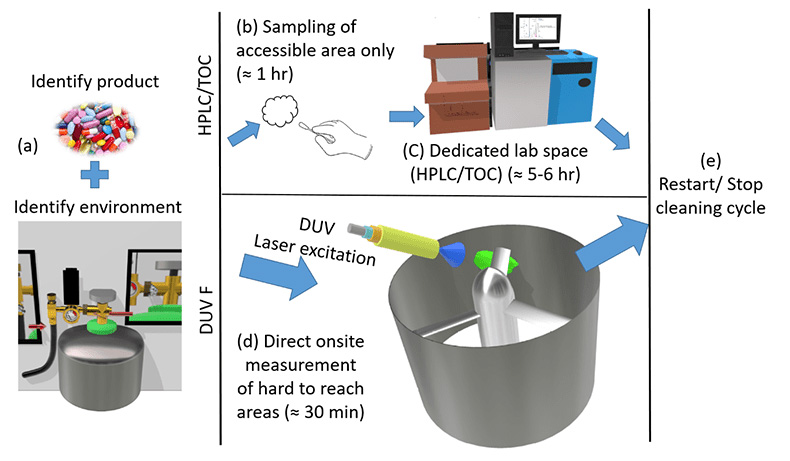
Figure 2: A schematic comparison of DUV laser-induced fluorescence measurement to traditional lab-based HPLC or TOC (total organic carbon) method. (a) The first stage of cleaning validation is identifying the product and the environment to be cleaned. (b) The traditional method requires sampling by swabbing or rinsing for obtaining samples and taken to the laboratories (c) measurement of the sample using a dedicated lab space, consuming ≈7−10 h for cleaning verification. (d) DUV laser-induced onsite fluorescence measurement is a single step process to verify cleaning in a short time (≈ 30 s) to advance to the (e) next step of the cleaning cycle.
Benefits of using Deep UV Raman Spectroscopy in Cleaning Validation
There are many benefits of using Deep UV Raman spectroscopy in cleaning validation, including rapid analysis and feedback on contaminant levels. Identity can be achieved with minimal sample preparation. Help to identify difficult to clean areas and simultaneous multi-component detection and reduced production downtime. It can also improve sample response times to prevent a measurement backlog.
One of the main advantages of using deep UV fluorescence spectroscopy for standoff trace detection is that there is very little interference from the background. The stray light used in the site would not interfere, since deep UV wavelengths used for generating fluorescence are to the shorter wavelength region in the electromagnetic spectrum. Techniques like near IR absorption when used for standoff or onsite detection can get high interference from the substrates like glass or polymer. DUV fluorescence spectroscopy has a high signal to noise ratio compared to any other onsite detection technologies on this regard.
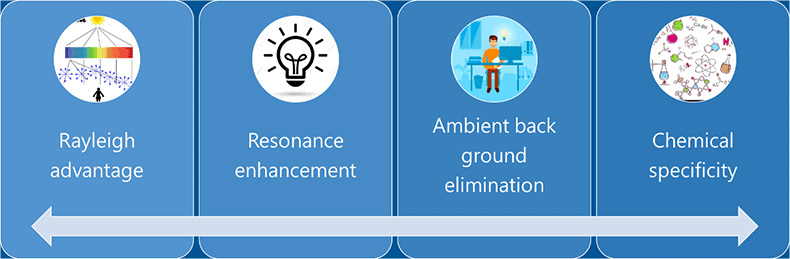
Figure 3: Advantages of using DUV for Raman scattering detection
CAPPA Expertise
CAPPA staff have over ten years’ experience working in the pharmaceutical industry with a specific focus on cleaning validation. CAPPA has a Deep UV Raman and fluorescence detection set up at 248.6nm, which is the only one of its kind in Ireland. CAPPA has worked with pharmaceutical companies including Janssen, Hooke Bio and Biocel.
You can learn more about Raman spectroscopy here and about CAPPA’s work in the pharmaceuticals sector here.
This post was first published on the CAPPA website.
